 [Image: A mobile biosafety containment apparatus in a simulated medivac exercise, via the CDC].
[Image: A mobile biosafety containment apparatus in a simulated medivac exercise, via the CDC].
Jonathan Richmond is director of Jonathan Richmond and Associates, Inc., a private biosafety consulting firm based in Atlanta, Georgia. He founded the company after nearly 35 years with the Centers for Disease Control.
The firm has a particular expertise in “facility design” for biosecure environments, with a strong emphasis on international biosafety education efforts. Richmond has worked on projects with NASA, the National Academy of Sciences, and the World Health Organization Biosafety Program, to name a few, and he has participated in supervisory visits to the remote bioweapons labs of the former Soviet Union.
As part of our ongoing series of quarantine-themed interviews, Nicola Twilley of Edible Geography and I spoke to Richmond about his firm’s work, about the technical specifics of biosafety, about the difference between biosecurity and quarantine, and about his own professional history.
BLDGBLOG: First of all, what exactly is biosafety? How is the term most commonly defined?
Jonathan Richmond: Biosafety is focused on issues related to microorganisms that cause diseases of humans or animals—and, if not the microorganisms themselves, the toxins that those organisms might produce. Achieving biosafety relies on a combination of the facility in which you’re going to work—that is, the engineering controls; the personal protective equipment that you’re able to wear; the medical surveillance programs that you’re involved with; whether you can be immunized against the disease agent, and so on—and then the practices and the procedures that you follow while you’re doing the work. The latter, ultimately, is perhaps the most important and most critical thing.
BLDGBLOG: Can you explain the difference between quarantine and biosafety?
Richmond: Quarantine is typically defined, or set up, when you have somebody who is already ill and you want to keep them from spreading their disease to other people. Our containment recommendations usually involve a range of biosafety practices, including quarantine. Some examples of that, of course, are all of the plans right now for the H1N1 swine flu. If you look at any of the websites that are talking about the precautions or procedures in college settings, for example, one of the things they emphasize is hand-washing—which is a practice or procedure that you can do to prevent the spread of a bug—but most go on to suggest that, if you are ill, then you should stay in your room. That’s a form of self-imposed quarantine.
We saw quarantine go into effect on a very large scale with the SARS virus outbreaks, primarily in SE Asia and Canada: there was an absolute need to isolate the people so that they did not spread the disease further.
Edible Geography: There seem to be different levels of biosafety. Someone staying in their dorm room is not biosecure, for instance; it’s just a form of social isolation.
Richmond: Yes, but that’s typically what one would do: keep the person isolated, either at home or, in the case of SARS, in hotel rooms, because they didn’t have appropriate isolation units in the hospitals.
It depends, I suppose, on the extent of a disease outbreak: how many people are actually infected? In a normal hospital setting, if you have only a single case, that person would be set up in what’s called an isolation area or isolation room. But as you get overwhelmed with cases, public health takes on other aspects, including trying to keep people quarantined in their own homes.
This was the standard set-up back at the turn of the last century. People would get various diseases going through the community, and public health people would go around and put a quarantine sign on your house. You were unable to have visitors; you weren’t supposed to go out. That’s basically the way things were contained.
 [Images: Biosafe labs and research facilities].
[Images: Biosafe labs and research facilities].
BLDGBLOG: Beyond these social and behavioral safeguards, what about the actual design of biosafe spaces?
Richmond: We look at four different levels of biocontainment.
Level one is basically no containment. Level one is for working with microorganisms that aren’t known to cause human illness. This is the kind of laboratory that, for example, you might see in a high school, or in an introductory course at the college level—even a lab that’s doing E. coli studies at your local sewer plant. It’s not much of anything at all, except simple behavioral guidelines—like don’t stick things in your mouth!
Level two is for working with microorganisms that are generally circulating in the community. Those are things that may cause illness; they’re probably the childhood diseases that everyone experienced early on, or that you’ve already been vaccinated against. In these particular laboratories, there’s a lot of emphasis on such things as hand-washing, wearing gowns, and wearing gloves. The facility itself is relatively simple; it’s more like a hospital laboratory. In the scope of things, this is where 90% or more of microbiological work is done. These labs are throughout the country and around the world.
With level three laboratories you begin to see some extraordinary engineering controls. These laboratories are the ones that are designed to work with microorganisms spread by the aerosol route. Level two are for ones that are spread by contact. At level three, you would see the engineering controls that give you things like directional-inward airflow. You would be looking for special filtration on exhaust air that’s leaving the labs. People would work inside biological safety cabinets, which are designed to protect the worker, to protect the product that you’re working on, and, indeed, to protect the environment – the micro-environment of the lab or the external environment. Those are really quite sophisticated in their design and in their operation. There is where a lot of work on, for instance, tuberculosis takes place in this country. A fair number of the organisms that are being considered as potential agents for bioterrorism are worked with at level three.
Then the level four laboratories are super-containment labs. They’re called maximum containment labs. These are labs where people would ordinarily wear a positive pressure suit while working—so they would have air supplied to them. The diseases they’re working with are the ones for which we really don’t have any vaccines and that, for the most part, we really don’t have any method to cure you if you get ill. These are the really dangerous pathogens—things like Ebola viruses, Marburg viruses, smallpox, etc.. At level four, you have bladder gaskets around the doors and you take chemical showers when you come out so that your suit gets decontaminated—and then you take that off, and you take a regular shower (which you generally would do at level three, also).
A lot of attention is also paid to the liquids at level three and four—collecting these and decontaminating them, either through chemical means or through heat treatment. Anything that comes out of a level three or four lab has to be decontaminated. Small objects—things like the used clothing, small animal carcasses, and used laboratory equipment—would be run through steam autoclaves. That will sterilize things. But the large volumes of liquid effluent that you might get, say, from showers, would be collected in a very large tank, typically directly underneath the laboratory, and then heat treated or chemically treated with chlorine. Heat treatment means that you take it up to very high temperatures for certain designated periods of time before you can then cool it down and release it into a sanitary sewer.
BLDGBLOG: I’d love to hear about two or three specific projects that you’ve worked on in the past. How did those projects foreground questions of biosafety or quarantine in an interesting or perhaps unexpected way?
Richmond: I used to work at CDC, and CDC is the only place in the United States—indeed, it’s one of only two places in the world—where you can work with smallpox virus. Smallpox was eradicated back in the 1970s and, at that time, a lot of work was done to make sure that any smallpox still held in laboratories was either destroyed or sent to CDC.
But the other laboratory where smallpox can be worked on is at an institute called Vector, out in Russia. It’s in Siberia. I had an opportunity to visit that particular laboratory a number of years ago, to see how our Russian colleagues had their containment system set up. This was so we would could compare it to how we have things set up in the U.S.
I was one of the very few Americans who were ever allowed inside that containment facility; it was an honor to go there and it was extraordinarily interesting.
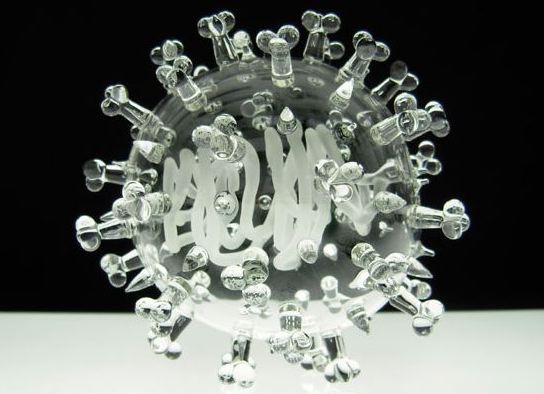
 [Images: From artist Luke Jerram’s extraordinary Glass Microbiology project].
[Images: From artist Luke Jerram’s extraordinary Glass Microbiology project].
Edible Geography: What differences did you notice in their set-up?
Richmond: The Vector labs, like so many things in Russia, are built on a much larger scale than in the U.S. Buildings seem to be bigger there, and the general way they do things—things are just bigger.
At the time I was there—and this would have been in the mid to late 90s—the control systems for things like airflow, which is so critical in these labs, was basically handled on a laptop computer in the U.S. Over there, however, it was all controlled by this big bank of flashing lights, and they had two or three people who simply sat there all day long and watched the flashing lights, making sure that they continued flashing. Their technology was not as far advanced.
Also, the suits they wore were of a slightly different design—but they accomplished the same thing. I actually thought it was a pretty good suit.
I’ve also had a chance, in the past, to work with NASA, as they began to think about sending people to Mars. If you remember, back when we sent people to the Moon, there was a concern that the astronauts might bring something back from outer space. Although they built some pretty robust facilities for it, the way they handled it was not quite the way we would do it today. In fact, I had a chance to visit the NASA facility where all of the moon rocks are stored. I was able to get inside the laboratory and fiddle around with some moon rocks.
But the work for the future was looking at new issues. It started with a straightforward question: what happens if we bring samples back from Mars? In that case, there was a dual concern. On the one hand, if the samples that came back happened to have an infectious agent in them, then we wanted to make sure we could protect the workers—and that’s pretty much the same way you would protect the workers in a level four facility.
But the other thing that NASA wanted to be very sure of was that we did not contaminate the samples with normal earth microorganisms. Because then you could say: oh, look, we found life on Mars! When, in reality, it was something that had been introduced once the sample got back.
So we got to talk about design concepts where you would have the same biosafety technology that we have here, such as negative air pressure, to prevent any organisms from escaping the facility. On the other hand, we would normally use positive air pressure to keep bugs out of a system, say, in a facility where you’re developing of vaccines or drugs. So we had to come up with a dual system that would allow for both positive and negative air pressure at the same time.

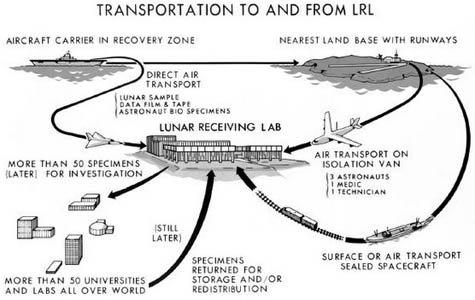 [Image: The returning Apollo astronauts relax inside their Airstream trailer/quarantine station, and their highly regulated route back from the moon].
[Image: The returning Apollo astronauts relax inside their Airstream trailer/quarantine station, and their highly regulated route back from the moon].
Edible Geography: How did you manage that?
Richmond: The concept designs that we developed used cabinets. Biological safety cabinets come in three basic formats: class one, two, and three. The class three cabinets are hermetically sealed devices; your arms go into the cabinet in these big gloves that are sealed to the cabinet itself. What the designers came up with was that, since the cabinet is normally operated under negative pressure, they put a second layer around the cabinet, and that would be under positive air pressure. You could do it other ways, obviously, but that was the one we came up with.
This design challenge then got extended by a project that we worked on through the National Academy of Sciences. They were thinking ahead to the point where the question became: How would we protect the astronauts if they went to Mars? How could we set up a laboratory there? That was very interesting, to learn about the geology and the geography of Mars, and to learn about some of the issues that we would have to deal with there.
For example, one of the biggest problems is the dust that just covers the surface of Mars. Regolith, they call it. If you’re running any kind of air-filtration system, it would very quickly clog if you had a dust storm going on.
Finally, I also had the opportunity to build a level three biocontainment laboratory in Africa—and that posed some very interesting questions. For the most part, in these developing countries, you don’t have all the things that we might expect to have, like running water or a reliable source of electricity. So the question there was: How do you design around those limitations?
Edible Geography: When you were working with NASA and the National Academy of Sciences, did concerns with quarantine also run the other way—in other words, quarantining materials from earth that we send to Mars, so as not to contaminate Mars?
Richmond: Yes. In fact, there’s a very interesting position in NASA; it’s called the Planetary Protection Officer. The person I met at the time who was Planetary Protection Officer was probably a combination of an engineer and a biologist—I don’t know what specific background he had. But that person oversees, and provides certain controls on, what is sent out from earth and what is returned to earth.
There are different criteria for this. For example, if a rocket is just going to go out, and there’s no intent for it to land anywhere—if it’s just going to send back information—then there’s less concern for what’s called “forward contamination.” But if we were to land that rocket on Mars, on an asteroid, or anywhere else, then there are things that we need to set up in order to sterilize a spacecraft before it can go.
Then, if it comes back, there are even more concerns. We spent a lot of time talking about how we could bring a rocket back from Mars. For instance, could it land on earth? Would they have to eject a capsule and parachute it down into the desert somewhere? How exactly could we do this? There was a lot of thought given to that.
The whole issue of quarantining samples, and bringing them back, also came up when the European Space Program wanted very much to be part of any Mars sample-return mission. In that case, if we can safely transport a sample from a containment lab somewhere in the U.S. to another lab in Europe, then could we also transport a subset of that sample to another country—say, to England—so that they could work on it over there?
We actually have developed some very robust mechanisms for the transport of infectious materials, globally, so I think the application of those same kinds of technologies to NASA sample-return missions would help assure us that we aren’t contaminating something that we’re shipping—or it wouldn’t break open and contaminate the world. The Andromeda Strain, you know.
BLDGBLOG: Is there a regulatory body that determines international standards of astronomical quarantine? For instance, what if China were to bring back a sample from Mars, but scientists in the U.S. thought it should be quarantined? How would this be regulated or enforced?
Richmond: Whether there’s such an agency or not, I don’t know. But I’ve been doing a lot of work in China recently, and in southeast Asia, and they are very concerned about biocontainment. They have pretty much adopted the same standards that we have in the U.S. The CDC’s book on biocontainment has actually been translated into at least seven languages, and it has pretty much become the accepted standard around the world.
So I think if you started to play around with something that you were bringing back from outer space… It’s such a small community of people, and they all have the same concerns. I am not terribly worried about the possibility of disagreement there.
 [Image: Biosafety cabinet and suited worker].
[Image: Biosafety cabinet and suited worker].
BLDGBLOG: I’m very interested in your own career trajectory, and in the nature of the private company that you’ve founded. Could you talk about the market niche—private biosafety consulting—that you stepped into with this?
Richmond: It was a pretty logical next step, when I left CDC after about 35 years of biosafety work, because I just have a wealth of knowledge and I didn’t want to let it all disappear. I’ve done a lot of publishing, and I’ve got a lot of stuff out there; but there’s a lot more to it that just comes from experience.
So we set up a very small company just to provide these services—either working with architects and engineers in the design phase, or even in the commissioning phases, or auditing the labs once their built, to make sure that they’re actually functioning the way they were intended.
We also do a lot of teaching for the people who work in the labs, and for the people who support the labs, to make sure that they understand how to work safely. It’s been very interesting to be out and about.
When I was at CDC, I also had lots of international travel experience. That means I’ve been able to work with ministers of health in different countries, or to work with them through different agencies.
Shortly after I retired, I spent three months at the World Health Organization working with them on developing their biosafety program. I was there about two weeks—maybe three weeks—when they said, listen, we have a SARS laboratory-acquired infection in China. We want you to go and investigate it. That was really neat, to be on the ground, doing that kind of work.
Edible Geography: So in that case, you were investigating a biosafety failure?
Richmond: Yes—we were looking to understand how it occurred. Was there one thing—or two things, or six things, or a convergence of things—that allowed for this to happen?
That’s actually something that we often do in the field. Every institution that has experienced a laboratory-acquired infection spends a lot of time trying to determine what went wrong so that we can spread the word. Was it a failure of equipment? Was it a failure of procedure? What exactly happened?
This, incidentally, is the model that was established by Arnold Wedum, who is considered to be the father of biosafety. Back in the 1940s, Wedum was a physician at Fort Detrick, Maryland; at that time they were studying offensive biological warfare. He had a small team of people and, any time something went wrong, they would spend a lot of time trying to figure out what happened. What was it that allowed for an agent to escape from the tube or from the centrifuge? That work continues today, following the same guidance document, in order to figure out what’s going on.
Edible Geography: How did you first get started in this field?
Richmond: Years and years ago, when I first started, I was at a place called the Plum Island Animal Disease Center. That’s located just off the tip of Long Island. We did a lot of what if? scenarios there—not just about Plum Island itself. For example, we were once talking about foot-and-mouth virus: how do you contain it? And how do you eradicate it?
It was an incident like that that got me out of the lab as a full-time virology researcher and into the field of biological safety. We had a breach of the containment at Plum Island back in the 1970s, and the director came to me and he said, “Richmond, we need a biosafety officer—would you like to be that?” And I said, “What’s that?” [laughs] Because the term biosafety officer really had not been coined until 1976 or thereabouts, at the Asilomar Conference in California, where the first recombinant DNA research was presented as scientific fact. The scientists there got quite concerned—almost frightened, I suppose—not knowing what this research was going to be lead to. They called for a moratorium on this type of work until the director of the National Institutes of Health could assure them that it was safe. It took about a year of research at NIH before they came up with the guidance document about biosafety levels.
But, in this document, it said that if you’re going to do this type of work then you need to have a biosafety officer. That’s how we got things started.
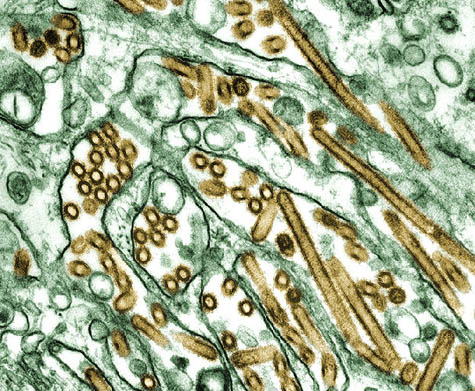 [Image: The H5N1 “bird flu” virus].
[Image: The H5N1 “bird flu” virus].
BLDGBLOG: As far as your current work goes, who, for the most part, is your clientele? In other words, are you working mostly for private firms or for state-affiliated clients?
Richmond: It’s some of everything. We’ve done a lot of work in Brazil. Brazil has built a very robust biosafety program over the last dozen years. I’ve done perhaps 20% of my work in southeast Asia—a lot of work in Singapore and China. In Singapore it’s all quasi-governmental, because of the nature of their dictatorship. And in China—who knows what it is. [laughter] But most of the work I’ve done there has been with CDC China—the equivalent of the CDC in Atlanta.
I also work with private industry—companies that say, hey, we’re doing this kind of work and we need someone from the outside to come by and take a look, to make sure we’re meeting the standards. Back in 1996, a program started at CDC that, by its very nature, fell into my lap. That was to look at what has been called the “Select Agent Program,” which is a bunch of microorganisms that have the potential for being used in biowarfare. Laboratories that are working with these agents have to be registered with the CDC or with the Department of Agriculture—or with both, depending upon the agent. In order to get that registration, or to keep that registration, they have to be inspected by CDC or USDA roughly once every three years. There’s a growing concern that some of these labs are not going to pass the inspection. So they contact me to do a pre-CDC inspection—we just help them to clear up the things that might stand in the way of getting, or not getting, their approval. That’s the kind of work that we do.
The stuff I like to do the most, though, is teaching. Getting out there and teaching people what we call the principles of biosafety—and, how, once they’ve learned those, they can get out and start teaching them to other people in turn.
To give you an example: in the mid-90s we did a program in Brazil called a “Course for Multipliers.” We had one representative from each of the seven federal laboratories, and one from each of the twenty-three state laboratories, and we gave them all a week of training. We gave them materials—Powerpoint presentations and books, all translated into Portuguese. Over the next three years, they went on to train 4,000 more people. That’s why I say that they have taken to this big time.
We’ve also done training in China, and we’ve been working with Pakistani and Indian folks, first of all to get biosafety associations started there but also to do some training. The Pakistanis have since started a biosafety association; the Indians are planning to, but they haven’t actually done so yet.
Edible Geography: Finally, what issues, innovations, or trends for biosafety do you see looking into the future?
Richmond: That’s an interesting question. A few years ago, the United States set out to build more containment laboratories. This actually started before 9/11, as we tried to get more hospitals to have their TB work done at level three, rather than at level two. But, then, of course, after 9/11, a lot of money got pumped into the system, and there have been a whole bunch of labs built since then, both level three and level four.
However, I also think we’re going to see more international growth of the field. I have a project I’ve been working on for the last five or six years now, trying to see if we can establish a standard for biosafety professionals that would be recognized globally. The World Health Organization recognizes 196 different countries, and probably only 20 of them have what you would consider a reasonable biosafety program.
I think we’re going to see this gradually grow. Our concept of the world is shrinking all the time, in terms of how quickly we can move and how quickly agents can move around. And these little bugs don’t carry passports and they don’t honor borders, and we have to be vigilant. We have to take a look at whatever’s coming down the pike next. The avian flu and, now, the H1N1—and who knows what it will be next year. But there’s more and more international cooperation on this kind of stuff, and I think it’s wonderful.
This autumn in New York City, Edible Geography and BLDGBLOG have teamed up to lead an 8-week design studio focusing on the spatial implications of quarantine; you can read more about it here. For our studio participants, we have been assembling a coursepack full of original content and interviews—but we decided that we should make this material available to everyone so that even those people who are not in New York City, and not enrolled in the quarantine studio, can follow along, offer commentary, and even be inspired to pursue projects of their own.
For other interviews in our quarantine series, check out Isolation or Quarantine: An Interview with Dr. Georges Benjamin, On the Other Side of Arrival: An Interview with David Barnes, The Last Town on Earth: An Interview with Thomas Mullen and Biology at the Border: An Interview with Alison Bashford.
Many more interviews are forthcoming.
